
Temporary High-Frequency Conductive Loss, Stable Cochlear Function
Phase 1 Audiological Outcomes of Intratympanic AC102
Overview
In this first-in-human phase 1 trial, we evaluated the audiological effects of intratympanic AC102, a novel inner-ear therapy delivered in a thermosensitive gel, in 42 normal-hearing volunteers. The main safety message is clear: AC102 is safe and well-tolerated. Furthermore, cochlear function remained intact, with no permanent sensorineural threshold shifts or deterioration in BERA or otoacoustic emissions.
From an audiological standpoint, the most notable finding was a short-lived, volume-dependent conductive hearing loss predominantly at high frequencies (4–8 kHz), reflecting a mechanical middle-ear effect of the injected gel rather than drug-related toxicity.
Key Audiological Findings
-
No permanent sensorineural changes: Bone conduction thresholds, BERA, and TEOAE/DPOAE remained stable throughout follow-up.
-
Transient high-frequency conductive loss: Air conduction thresholds increased temporarily, most clearly between 4 and 8 kHz, and returned to baseline within days.
-
Volume-dependent effect, independent of AC102: The magnitude of the conductive shift scaled with injected volume (50–800 μl) and occurred for both placebo gel and AC102, indicating a mechanical middle-ear effect rather than drug toxicity.
-
Mild, procedure-related symptoms only: Transient ear discomfort, brief vertigo, tinnitus, and minor bleeding at the injection site were observed, resolving within about a week.
Volume–Effect Relationship (FIG. 4)
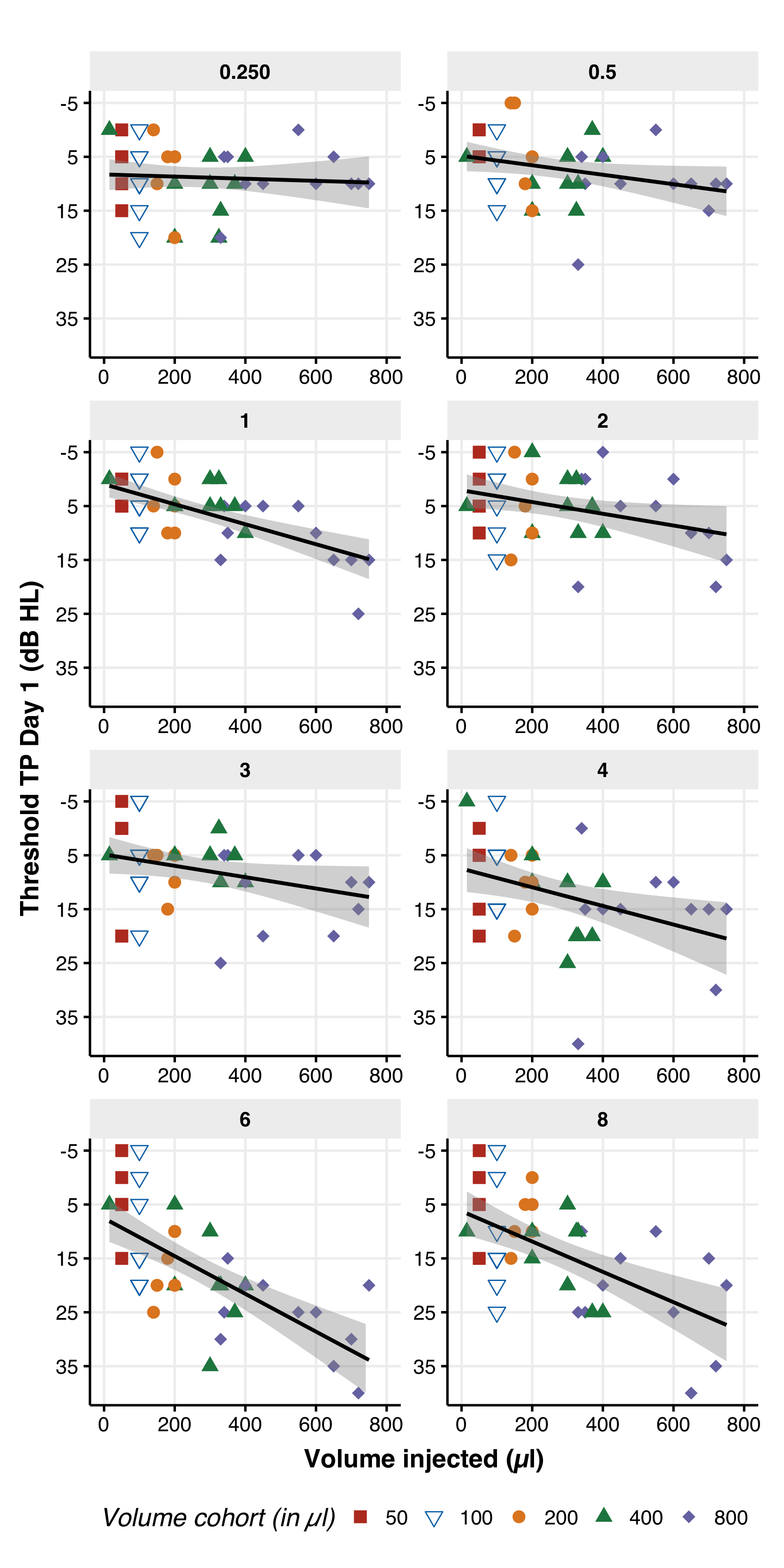
Figure 4. The average air conduction threshold versus the injected volume of either placebo or AC102 on the day of injection. Each panel showcases the frequency tested, ranging from 0.25 to 8.0 kHz, and displays the threshold measured after injection for the injected ear (threshold at TP day 1 in dB HL). The color code in each panel represents the volume cohorts, with red representing 50 μl, blue/white triangle representing 100 μl, orange circle representing 200 μl, green triangle representing 400 μl, and purple diamond representing 800 μl. The x-axis displays the exact volume injected for each participant, and the y-axis shows the effect of the injected volume on the threshold, with the most significant impact seen between 4 and 8 kHz. For each frequency, a linear fit with the confidence interval around the mean is presented (fit in black, confidence interval in gray).
Take-Home for Audiology
- Intratympanic AC102 in a thermosensitive gel can be delivered without permanent cochlear damage, as evidenced by stable BC, BERA, and OAEs.
- Clinically, you can expect a short-term, high-frequency air–bone gap whose size depends on the injected volume and resolves within days.
- Patients should be counseled about transient “muffled” high-frequency hearing and mild procedure-related discomfort, but reassured that long-term thresholds are not adversely affected.
- A phase 2 trial is currently ongoing, focusing on the efficacy of AC102 in sudden sensorineural hearing loss (SSNHL). Because of the tight time window after onset, our local recruitment is modest, but we are closely involved in the clinical and methodological design.
- After many negative trials and a few promising successes (e.g. gene-specific approaches such as OTOF), the field urgently needs an effective treatment for SSNHL to mitigate the profound impact that patients report in daily life.
Cris
RESEARCH · INNER-EAR-THERAPY · CLINICAL-TRIALS
AC102 intratympanic conductive hearing loss sudden sensorineural hearing loss thermosensitive gel phase 1 trial